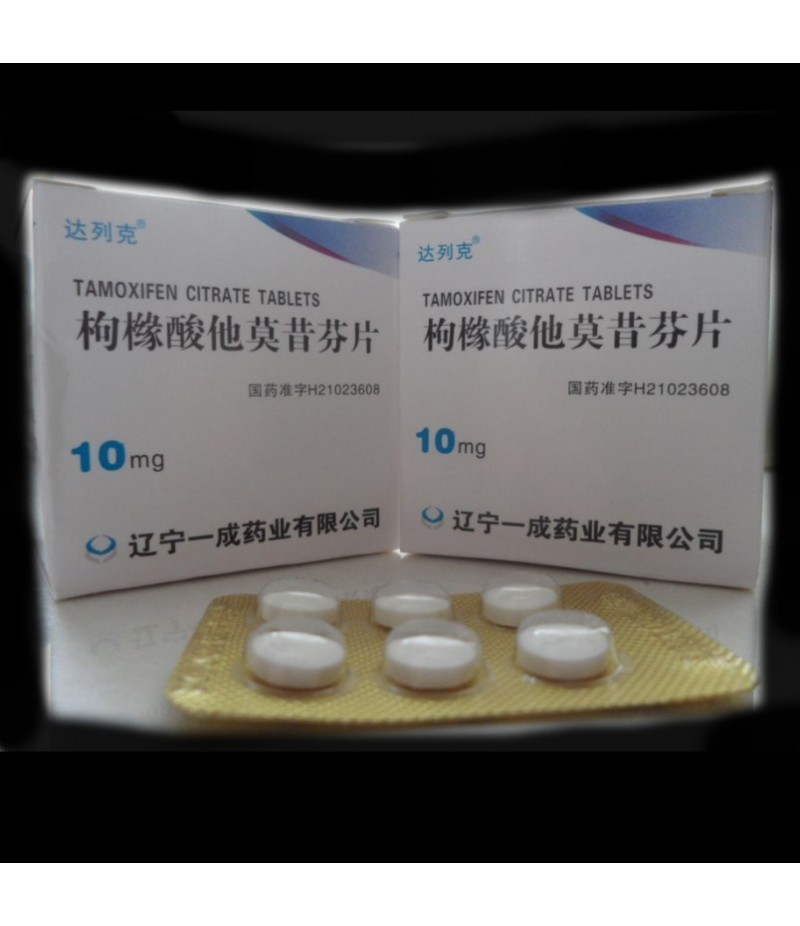
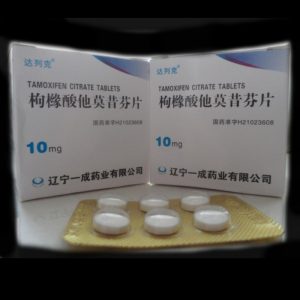
Nolvadex (tamoxifen citrate) is used to treat breast cancer that has spread to other parts of the body (metastatic breast cancer), to treat breast cancer in certain patients after surgery and radiation therapy, and to reduce the chances of breast cancer in high-risk patients.
Most common side effects include hot flashes, nausea, leg cramps, hair thinning, or headache. A loss of sexual ability/interest may occur in men.
Other less common side effects may also occur.
DETAILS
The recommended daily dose of Nolvadex for patients with breast cancer is 20-40 mg per day, in tablet form. Patients taking anastrozole or letrozole should not take Nolvadex as serious interactions could occur. SSRI antidepressants and cimeditdine may affect how Nolvadex works. Patients taking coumarin-type anticoagulants should be closely monitored. There may be risks to the fetus if Nolvadex is taken by pregnant women, however the benefit of the drug may warrant its use despite the potential risks. Nolvadex has been reported to inhibit lactation. It is not known whether the medication is passed through breast milk, but because of the potential risks for the fetus, women who are taking Nolvadex should not breast feed.
Patients receiving anastrozole had an increase in joint disorders (including arthritis, arthrosis and arthralgia) compared with patients receiving NOLVADEX (tamoxifen citrate) . Patients receiving anastrozole had an increase in the incidence of all fractures (specifically fractures of spine, hip and wrist) [315 (10%)] compared with patients receiving NOLVADEX (tamoxifen citrate) [209 (7%)]. Patients receiving anastrozole had a decrease in hot flashes, vaginal bleeding, vaginal discharge, endometrial cancer, venous thromboembolic events and ischemic cerebrovascular events compared with patients receiving NOLVADEX (tamoxifen citrate) .
Patients receiving NOLVADEX (tamoxifen citrate) had a decrease in hypercholesterolemia (108 [3.5%]) compared to patients receiving anastrozole (278 [9%]). Angina pectoris was reported in 71 [2.3%] patients in the anastrozole arm and 51 [1.6%] patients in the NOLVADEX (tamoxifencitrate) arm; myocardial infarction was reported in 37 [1.2%] patients in the anastrozole arm and in 34 [1.1%] patients in the NOLVADEX (tamoxifencitrate) arm.
Results from the adjuvant trial bone substudy, at 12 and 24 months demonstrated that patients receiving anastrozole had a mean decrease in both lumbar spine and total hip bone mineral density (BMD) compared to baseline. Patients receiving NOLVADEX (tamoxifen citrate) had a mean increase in both lumbar spine and total hip BMD compared to baseline.
The type and frequency of adverse events in the NSABP B-24 trial were consistent with those observed in the other adjuvant trials conducted with NOLVADEX (tamoxifen citrate) .
In the NSABP P-1 Trial, there was an increase in five serious adverse effects in the NOLVADEX (tamoxifen citrate) group: endometrial cancer (33 cases in the NOLVADEX (tamoxifen citrate) group vs. 14 in the placebo group);pulmonary embolism (18 cases in the NOLVADEX (tamoxifen citrate) group vs. 6 in the placebo group); deep vein thrombosis (30 cases in the NOLVADEX (tamoxifen citrate) group vs. 19 in the placebo group); stroke (34 cases in the NOLVADEX (tamoxifen citrate) group vs. 24 in the placebo group); cataract formation (540 cases in the NOLVADEX (tamoxifen citrate) group vs. 483 in the placebo group) and cataract surgery (101 cases in the NOLVADEX (tamoxifen citrate) group vs. 63 participants receiving NOLVADEX (tamoxifen citrate) and placebo therapy, respectively withdrew from the trial for medical reasons. The following are the medical reasons for withdrawing from NOLVADEX (tamoxifen citrate) and placebo therapy, respectively: Hot flashes (3.1% vs. 1.5%) and Vaginal Discharge (0.5% vs.0.1%).
In the NSABP P-1 trial, 8.7% and 9.6% of participants receiving NOLVADEX (tamoxifen citrate) and placebo therapy, respectively withdrew for non-medical reasons.
On the NSABP P-1 trial, hot flashes of any severity occurred in 68% of women on placebo and in 80% of women on NOLVADEX (tamoxifen citrate) . Severe hot flashes occurred in 28% of women on placebo and 45% of women on NOLVADEX (tamoxifen citrate) . Vaginal discharge occurred in 35% and 55% of women on placebo and NOLVADEX (tamoxifen citrate) respectively; and was severe in 4.5% and 12.3% respectively. There was no difference in the incidence of vaginal bleeding between treatment arms.
Mean uterine volume increased after 6 months of treatment and doubled at the end of the one-year study. A causal relationship has not been established; however, as an increase in the incidence of endometrialadenocarcinoma and uterine sarcoma has been noted in adults treated with NOLVADEX (see *BOXED WARNING*), continued monitoring of McCune-Albright patients treated with NOLVADEX (tamoxifen citrate) for long-term effects is recommended. *The safety and efficacy of NOLVADEX (tamoxifen citrate) for girls aged two to 10 years with McCune-Albright Syndrome and precocious puberty have not been studied beyond one year of treatment. The long-term effects of NOLVADEX (tamoxifen citrate) therapy in girls have not been established.
Less frequently reported adverse reactions are vaginal bleeding, vaginal discharge, menstrual irregularities, skin rash and headaches. Usually these have not been of sufficient severity to require dosage reduction or discontinuation of treatment. Very rare reports of erythema multiforme,Stevens-Johnson syndrome, bullous pemphigoid, interstitial pneumonitis, and rare reports of hypersensitivity reactions including angioedema have been reported with NOLVADEX (tamoxifen citrate) therapy. In some of these cases, the time to onset was more than one year. Rarely, elevation of serum triglyceride levels, in some cases with pancreatitis, may be associated with the use of NOLVADEX (tamoxifen citrate).





Reviews
There are no reviews yet.